We take novel approaches to defined disease pathways and leverage our metabolic expertise to develop therapies that have the potential to overcome the shortcoming of current standards of care by significantly improving clinical outcomes for patients.
| Program | IND | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
| RZ358 | Congenital hyperinsulinism Orphan designation in US and EU Pediatric Rare Disease designation in US | ||||
| Drug | Stage |
|---|---|
| RZ358 | Orphan designation in US and EU Pediatric Rare Disease designation in US Congenital hyperinsulinism | Phase 3 |
RZ358
Congenital hyperinsulinism (HI) is a rare pediatric genetic disorder characterized by excessive production of insulin by the pancreas. If left untreated, elevated insulin levels can cause extreme hypoglycemic (low blood sugar) events, increasing the risk of neurologic and developmental complications, including persistent feeding problems, learning disabilities, recurrent seizures, brain damage or even death.
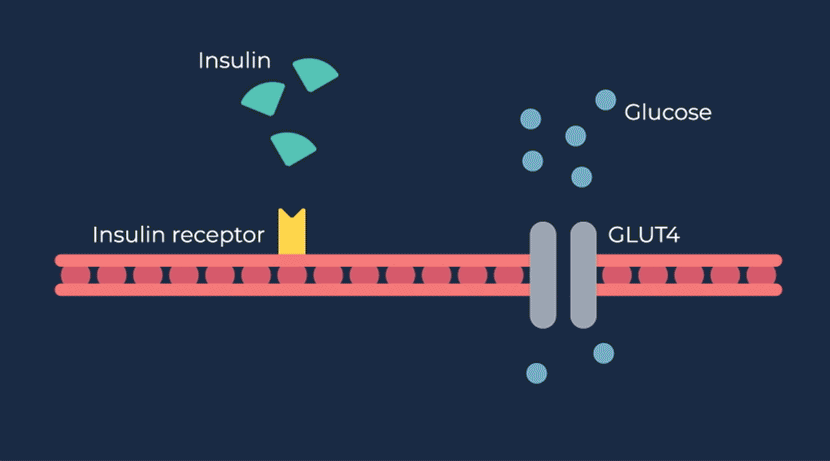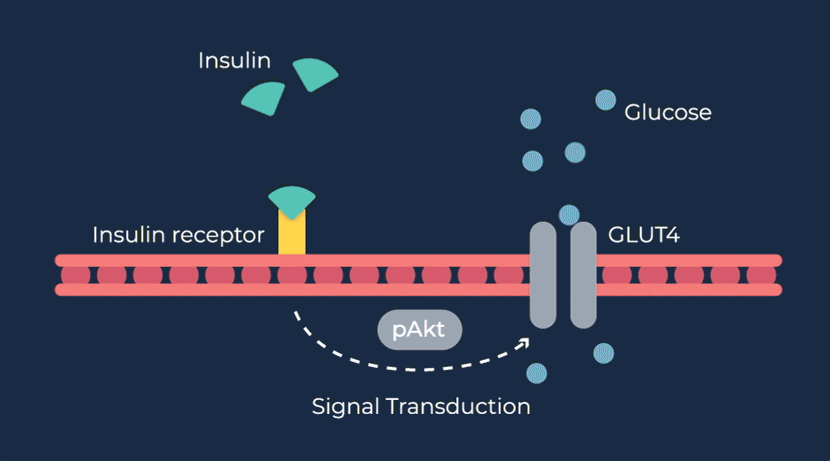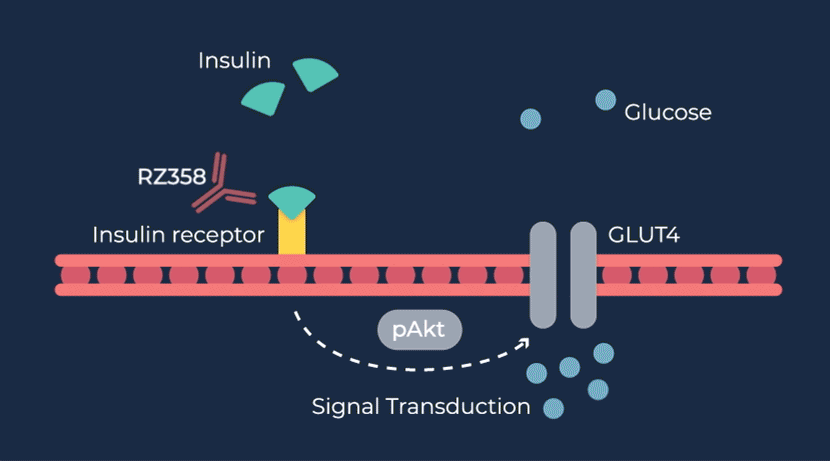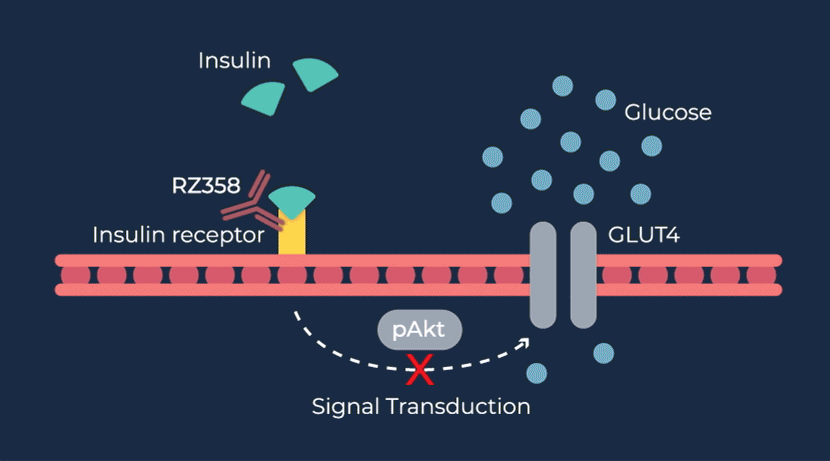
RZ358 is a fully human monoclonal antibody that works downstream from the pancreas and other sources of insulin or related paraneoplastic substances, and instead binds to a unique allosteric site on insulin receptors in the liver, fat, and muscle. The antibody counteracts excess insulin receptor activation by insulin and other effector substances (such as IGF-2), thereby improving hypoglycemia. Because RZ358 acts downstream from the pancreas at the insulin receptor, it has the potential to be universally effective at treating hypoglycemia due to congenital HI, regardless of the causative genetic defect, as well as acquired forms of HI such as those mediated by insulinomas (ICTs) and other tumor types (NICTs).
Publications
The birth prevalence of congenital hyperinsulinism: a narrative review of the epidemiology of a rare disease
Read MoreAn Analysis of Overnight Hypoglycemia in Patients with Congenital Hyperinsulinism:Results from the RZ358-606 (RIZE) StudyWhile
Read MoreClinical Studies
Rezolute recently revealed results from the Phase 2b study. View Results
x close
| Program | IND | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
| RZ358 | Tumor Hyperinsulinism Expanded Access Program | ||||
| Drug | Stage |
|---|---|
| RZ358 | Expanded Access Program Tumor Hyperinsulinism | Phase 2 |
RZ358
Tumor HI may be caused by two distinct types of tumors: islet cell tumors (ICTs) and non-islet cell tumors (NICTs), both of which lead to hypoglycemia due to excessive activation of the insulin receptor. Insulinomas are the most common type of functional ICT and cause hypoglycemia because of over production of insulin. NICTs can cause hypoglycemia by producing and secreting insulin-like paraneoplastic substances such as IGF-2 or related variants that bind to and activate the insulin receptor. This form of hypoglycemia can occur in more than 15 different tumor types, 60 percent of which are malignant, including hepatocellular carcinoma. The total addressable market for the combined indications causing tumor HI is estimated to be approximately 4,500 patients in the U.S. alone, including approximately 1,500 with ICTH and 3,000 with NICTH.
The unique mechanism of action of RZ358 to attenuate excess insulin receptor activation mediated by insulin and related substances makes the therapy a potential universal treatment for hypoglycemia resulting from any cause of hyperinsulinism. Under the Expanded Access Program, four individuals with ICTH have been successfully treated with RZ358 resulting in substantial hypoglycemia improvement, discontinuation of intravenous dextrose, and hospital discharge. Rezolute has received alignment with the FDA to initiate a late-stage registrational study in patients with hypoglycemia due to tumor HI, which the company is exploring to potentially include the NICTH population in addition to ICTH patients.
| Program | IND | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|---|
| RZ402 | Diabetic Macular Edema | ||||
| Drug | Stage |
|---|---|
| RZ402 | Diabetic Macular Edema | Phase 2 |
RZ402
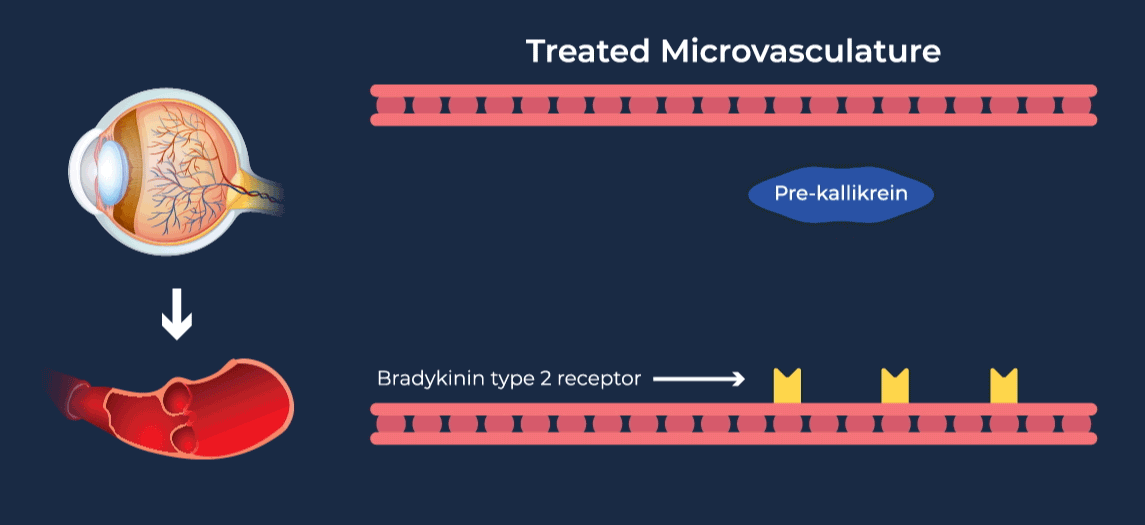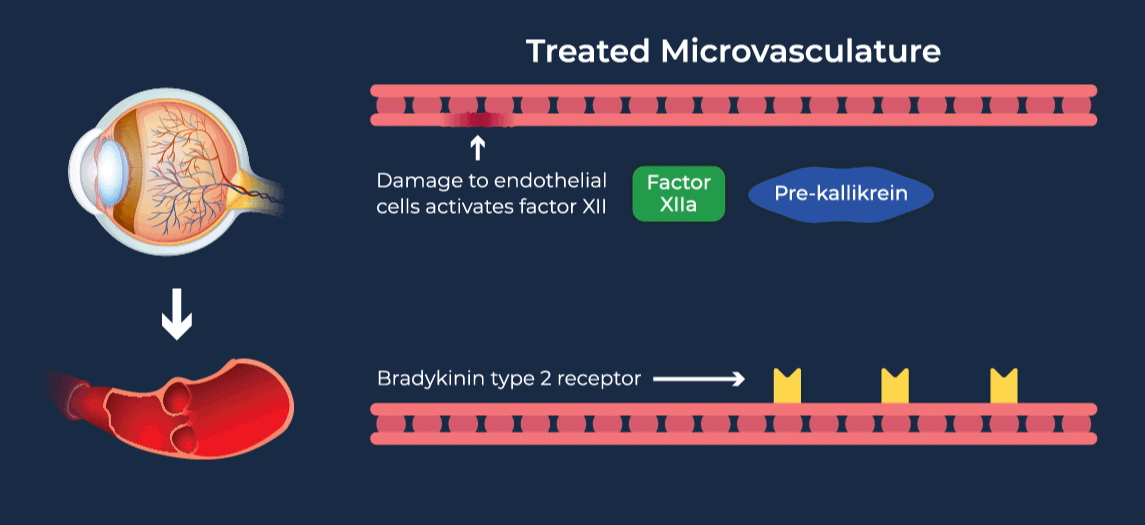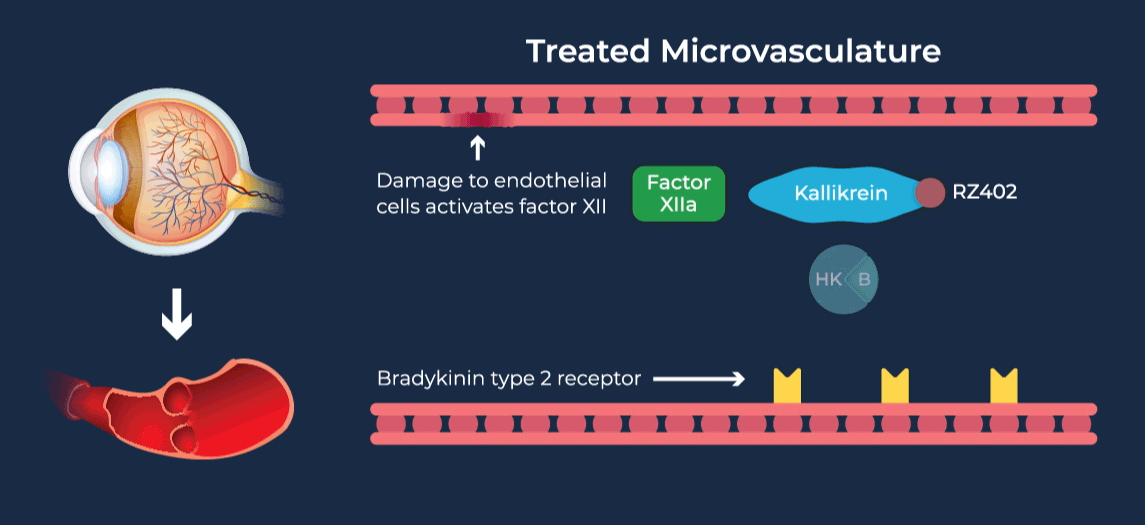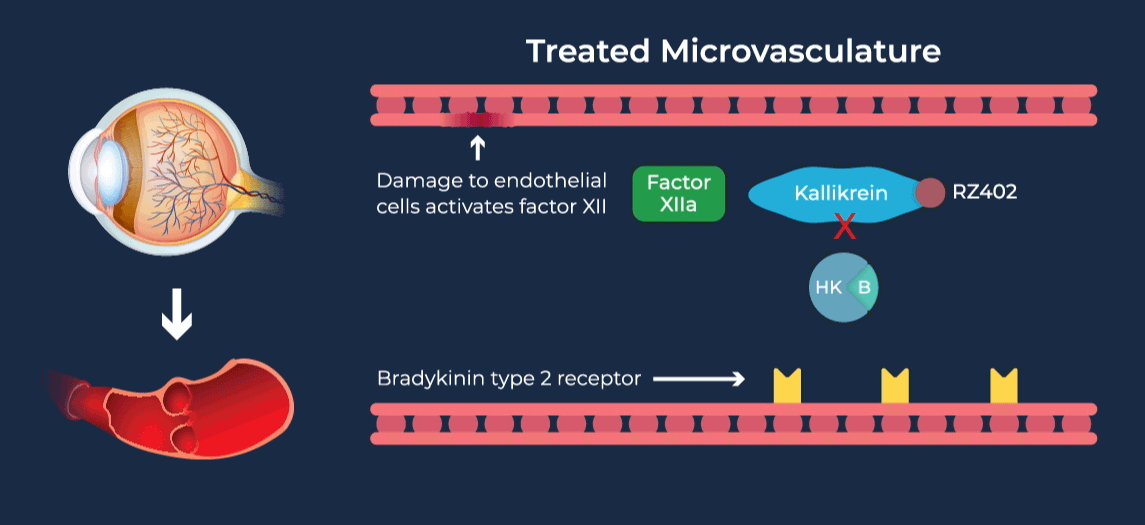
Diabetic retinopathy (DR) affects approximately one third of adults with diabetes and is the leading cause of vision loss in the working age population. Diabetic Macular Edema (DME) is a severe vision-threatening complication of DR characterized by swelling of the retina and thickening of the macula, the part of the eye that is responsible for high-resolution vision. Anti-vascular growth factor (anti-VEGF) injections into the eye are the current standard of care for DME, requiring continued administration over long periods of time to preserve vision. Due to their invasive route of administration and occasional serious side effects, there is a tendency to delay treatment until later in the disease course, and long-term compliance with eye injection regimens can be difficult for patients. Coupled with inadequate responsiveness in some patients, this leads to overall undertreatment and suboptimal vision outcomes in DME patients.
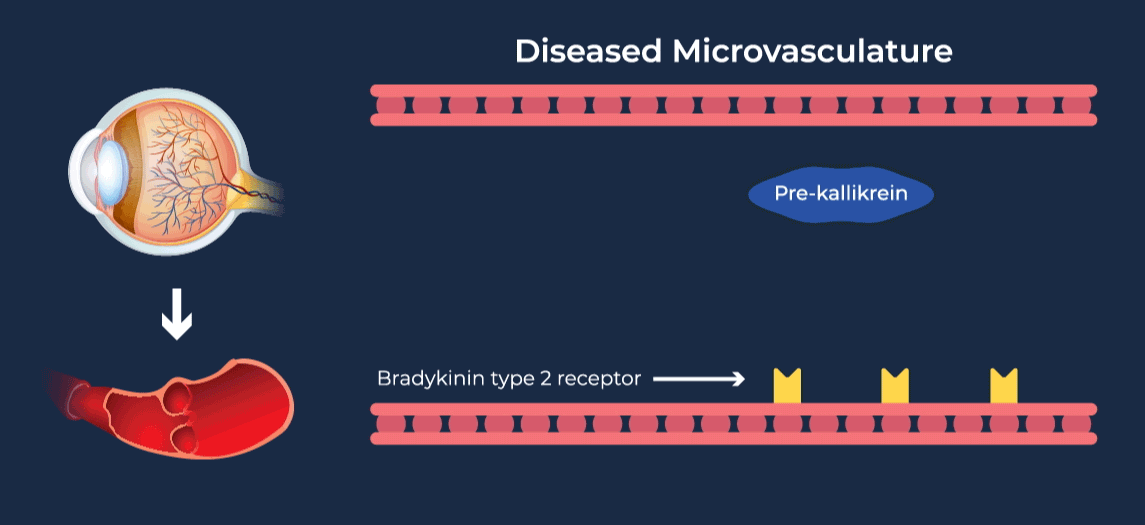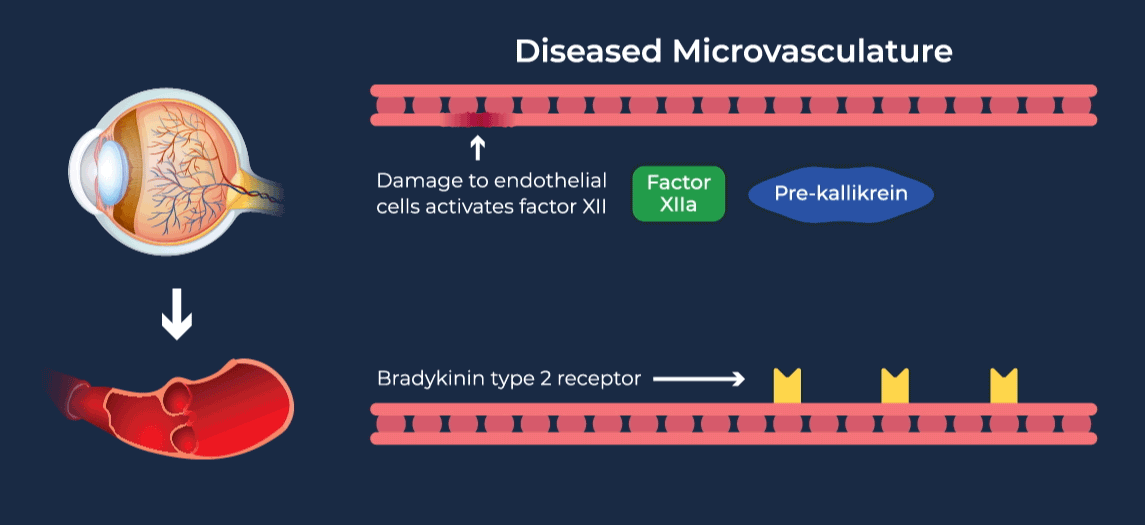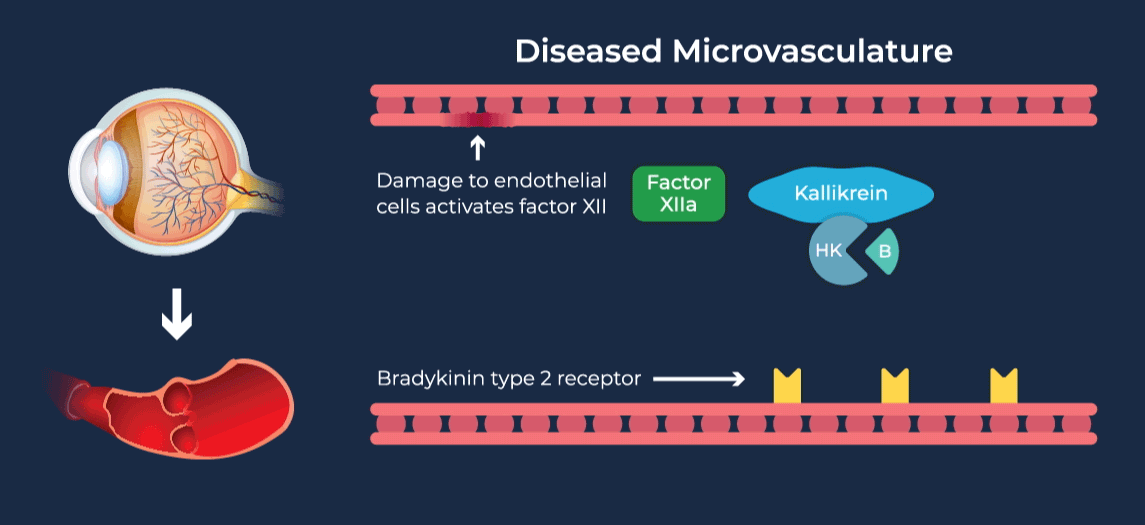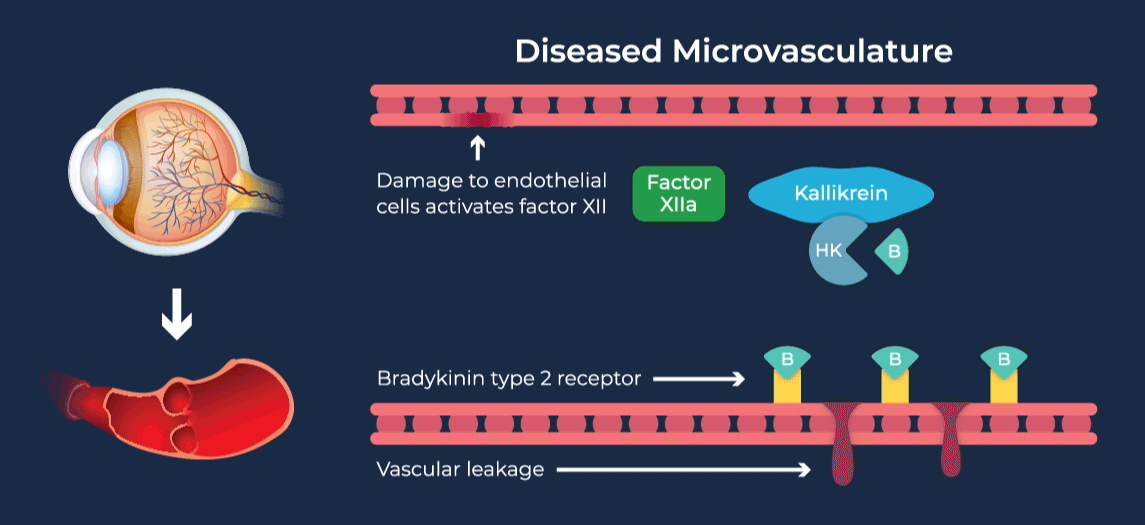
The contact-activation kallikrein-kinin system promotes increased vascular permeability and inflammation via key downstream mediators, including bradykinin, and activation of the intrinsic pathway of coagulation. Pathophysiologic upregulation of this system has been linked to a variety of diseases which are characterized by vascular dysfunction, including diabetic macular edema.
RZ402 is a small molecule selective and potent plasma kallikrein inhibitor (PKI) being developed as a potential oral therapy for the chronic treatment of diabetic macular edema (DME). By inhibiting the formation of kallikrein, RZ402 is designed to block downstream bradykinin production and the pro-inflammatory, pro-coagulant, and fluid-leakage contact-activation cascade.
RZ402 has been shown to reduce and prevent retinal vascular leakage in animal models by 80-90%.
Publications
Plasma Kallikrein Mediates Angiotensin II Type 1 Receptor–Stimulated Retinal Vascular Permeability
Read MoreNonclinical safety and pharmacology of RZ402, a plasma kallikrein inhibitor, for the treatment of diabetic macular edema as a daily oral therapy
Read MoreClinical Trials
RZ402, our oral PKI in development for DME, recently announced results from the Phase 1b study. View Results
RZ402, our oral PKI in development for DME, recently announced results from the Phase 1b study. View Results
Rezolute initiated a Phase 2a study in December 2022, with announcement of top line data planned for early 2024.
x close


